Particle-Hemodynamics and Biomechanics
The modern trend for effective medical treatment is patient-specific intervention; because, it provides best-possible health care and minimizes post-treatment complications. For example, as part of the smart inhaler system (SIS) project we will deal with patient-specific lung morphologies to determine optimal SIS operational parameters in order to achieve targeted drug-aerosol delivery on an individual basis. Another patient-specific paradigm application is liver-tumor targeting with radioactive micro spheres and or chemo-drugs.


To improve the treatment of unresectable tumors, a direct tumor-targeting methodology has been proposed in which precise catheter positioning, particle velocity, and infusion interval allows for the injected therapeutic particles to be transported by the blood flow to the target site. As shown below, we have established the targeting methodology for micron particles in hepatic arterial systems.
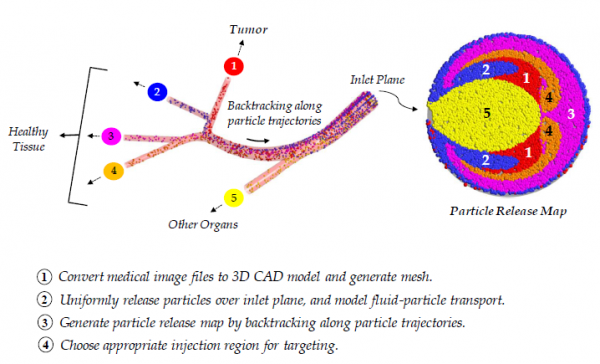
The following link demonstrates experimental validation of our direct-tumor targeting methodology.
Experimental Proof of Concept
Direct Multifunctional Nanoparticle Targeting
While direct tumor-targeting has been demonstrated for micron particles, it has yet to be established for nanoparticles in an arterial system. This is important to independently demonstrate due to the possible effects of Brownian diffusion (due to the bombardment of fluid molecules) or shear-induced diffusion (due to interactions with blood cells). As evidenced by the large Peclet number for the given particle size and main flow field (i.e., greater than 104), the transport in the first few generations of the hepatic arterial system (where targeting will take place) may be advection dominant. However, diffusion may become a dominant mechanism near the wall (where the velocity can become quite small) or in areas where the shear rate is large. So, we developed novel computer simulation models to study nanoparticle transport in a representative human hepatic artery system, subject to shear-induced diffusion of nanoparticles due to hydrodynamic interactions with red blood cells. The following figures are examples of targeting with multifunctional nanoparticles where the developing nanodrug-streams are targeted to artery branches D1 and D3 to which a tumor may be connected.
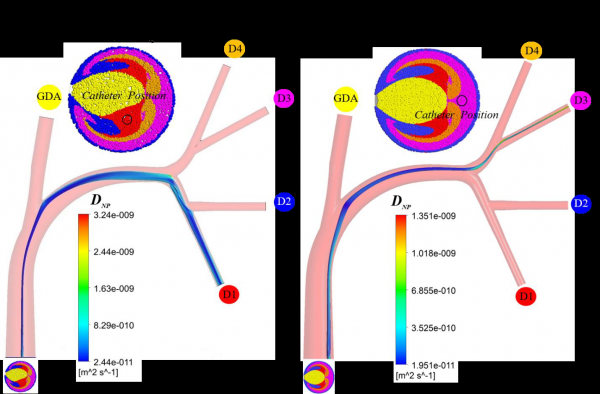
With the ability to target specific downstream daughter vessels, the direct tumor-targeting methodology is expected to revolutionize cancer therapy by minimizing side-effects, allowing for increased drug dosage, and potentially broadening the candidacy pool for these life-saving procedures. Most importantly, this investigation may better inform physicians on the importance of catheter placement and injection conditions in a wide range of local intra-arterial infusion therapies.